Innovating for kids with HIV
How Uganda is rolling out new technologies that can help transform the global paediatric HIV response
1 December 2019
A mother's journey
Rays of sunlight come through a small window of a mudbrick house as Ruth wakes up with her two daughters Lighton and Joanita in Mbarara, a city in the southwestern region of Uganda. As always, it's giggles and smiles as the young mother plays with her children. But at 7am her daily routine becomes no laughing matter – she must give treatments to her daughters, both of whom are living with HIV.
The medicines are given daily. By staying on these 'antiretroviral' treatments, her daughters can live full, healthy lives and their HIV can be managed as a chronic illness. Ruth knows this and never misses a day’s dose.

The kids take their medicines with food Ruth prepares and then they set off. Today they are travelling to the hospital for their HIV care. They head into the hustle and bustle of one of the main roads of Mbarara, and hail a 'boda boda,' a motorcycle-taxi service commonly used in Uganda.
It is a journey that mothers caring for children with HIV make throughout Africa. Many of the almost two million children living with HIV are in similar communities in sub-Saharan Africa: on the outskirts of town, in rural settings, or in deeply remote villages a day’s trip from the nearest hospital.
It is a journey that is wrought with difficulties, as mothers grapple with stigma, limited resources, and the everyday challenges of travelling long distances to access testing and treatment for HIV.

Ruth heads out to the hospital with her family
Ruth heads out to the hospital with her family
But it is a journey that is getting easier now in some countries, in part due to innovations that are being rolled out in places like Mbarara Regional Referral Hospital. This large hospital serves residents of this hilly region on the border of Rwanda, which includes a large refugee camp. For some years now, Mbarara hospital has been at the centre of new technologies rolled out by the Ugandan government and its partners, many financed by the global health initiative Unitaid.
On the one hand, the introduction of new “point-of-care” HIV diagnostic technologies now allow infants and young children to be tested rapidly and accurately. Supported by UNICEF and the Clinton Health Access Initiative Inc., these technologies are being used to great effect at Mbarara. On the other hand, new child-friendly HIV treatments are being tested and implemented in Mbarara by the not-for-profit Drugs for Neglected Diseases initiative (DNDi) and the Indian generic company Cipla, allowing caregivers to access better treatment for kids with HIV.
See the progress being made in Mbarara:
Although these technologies are transforming lives, much work remains to end the long-standing neglect of HIV-exposed children, including children living with HIV.
Why have the needs of kids with HIV been so neglected?
Children slipping through the cracks
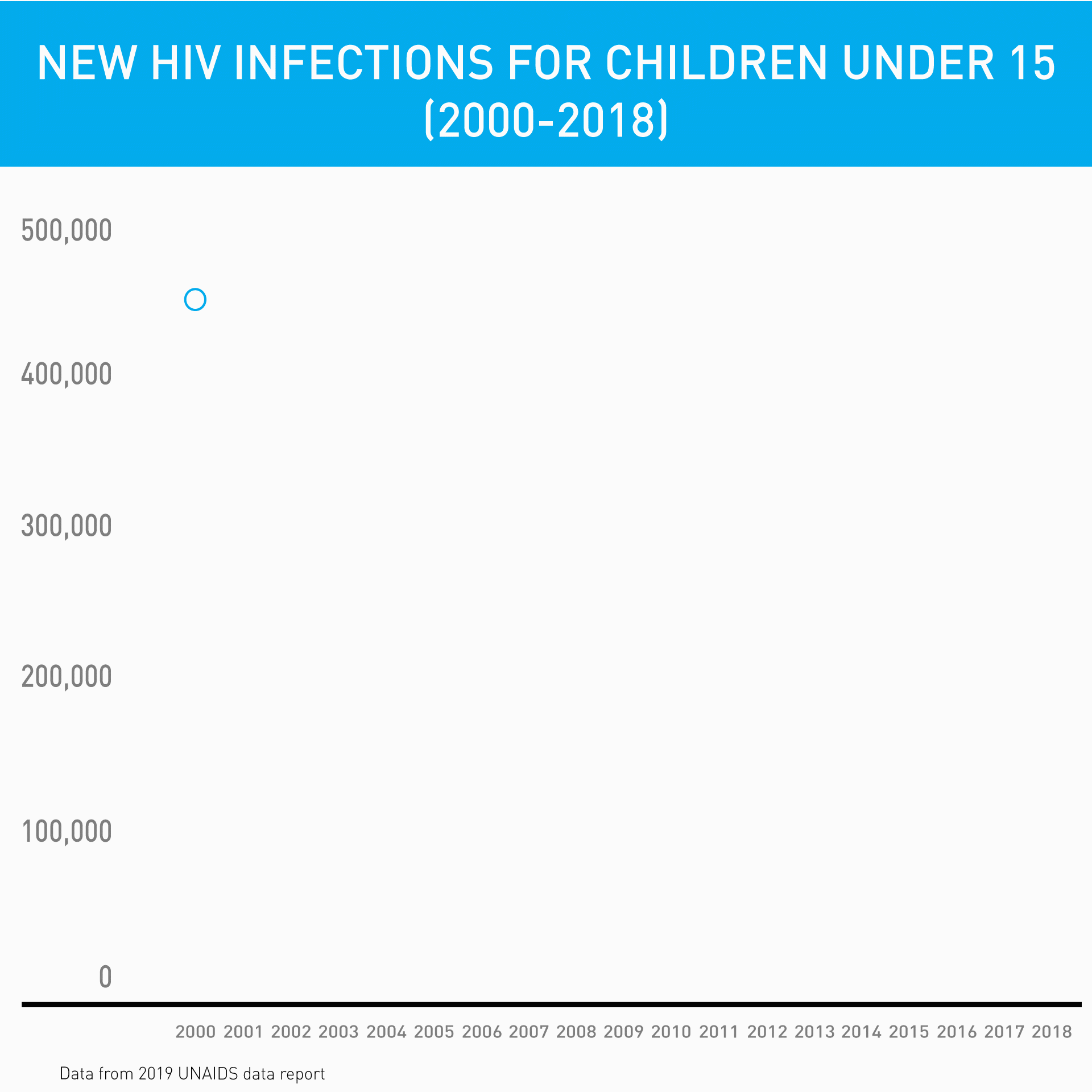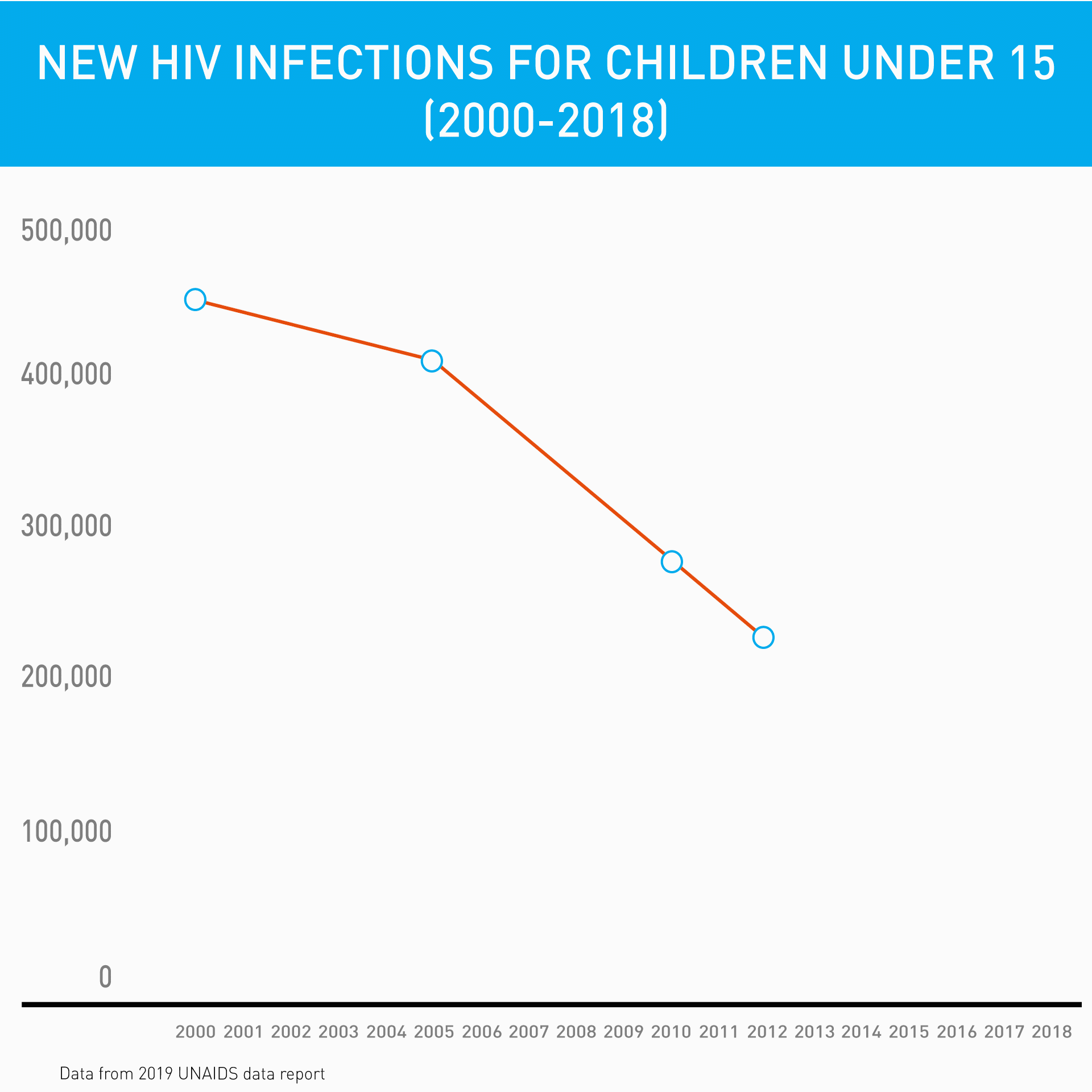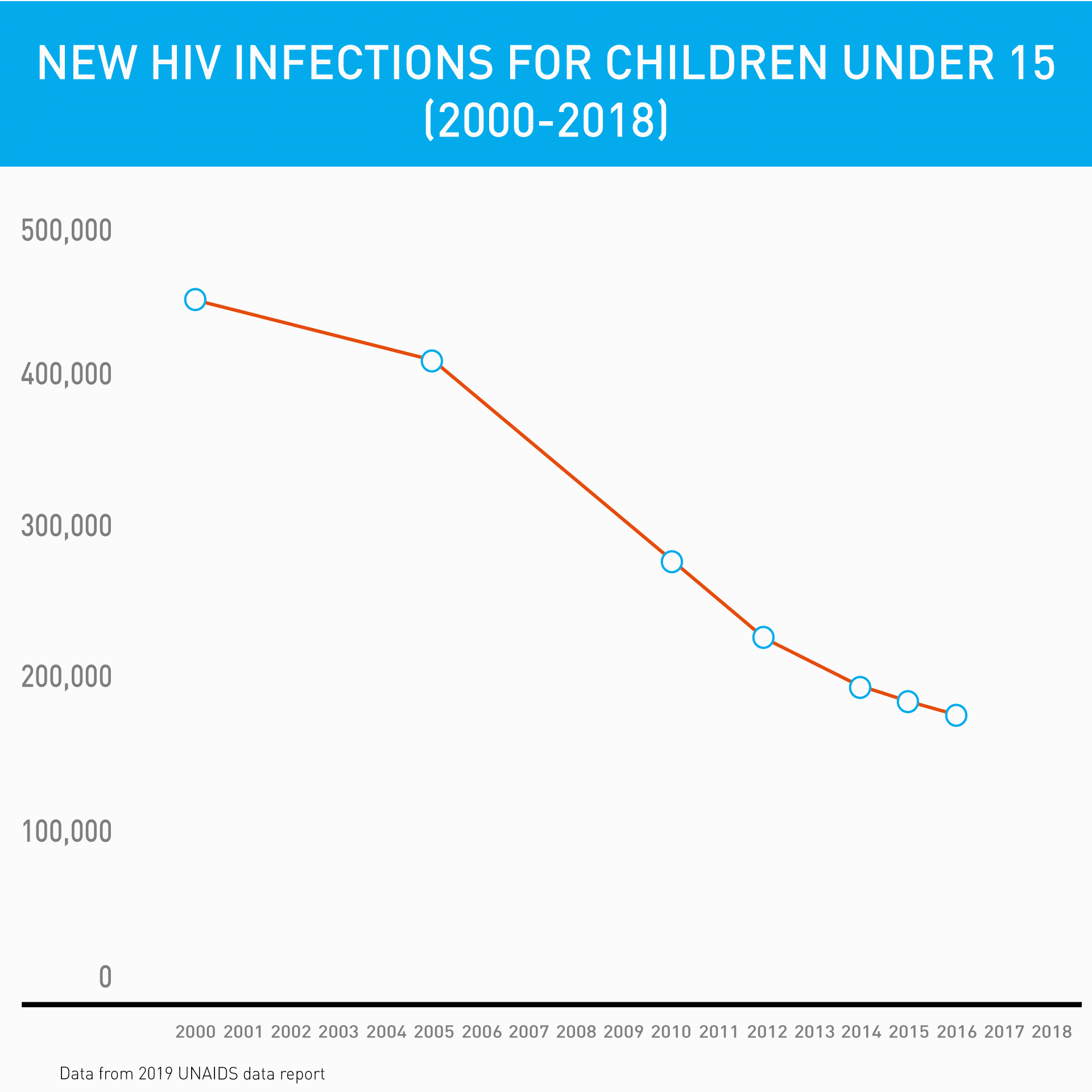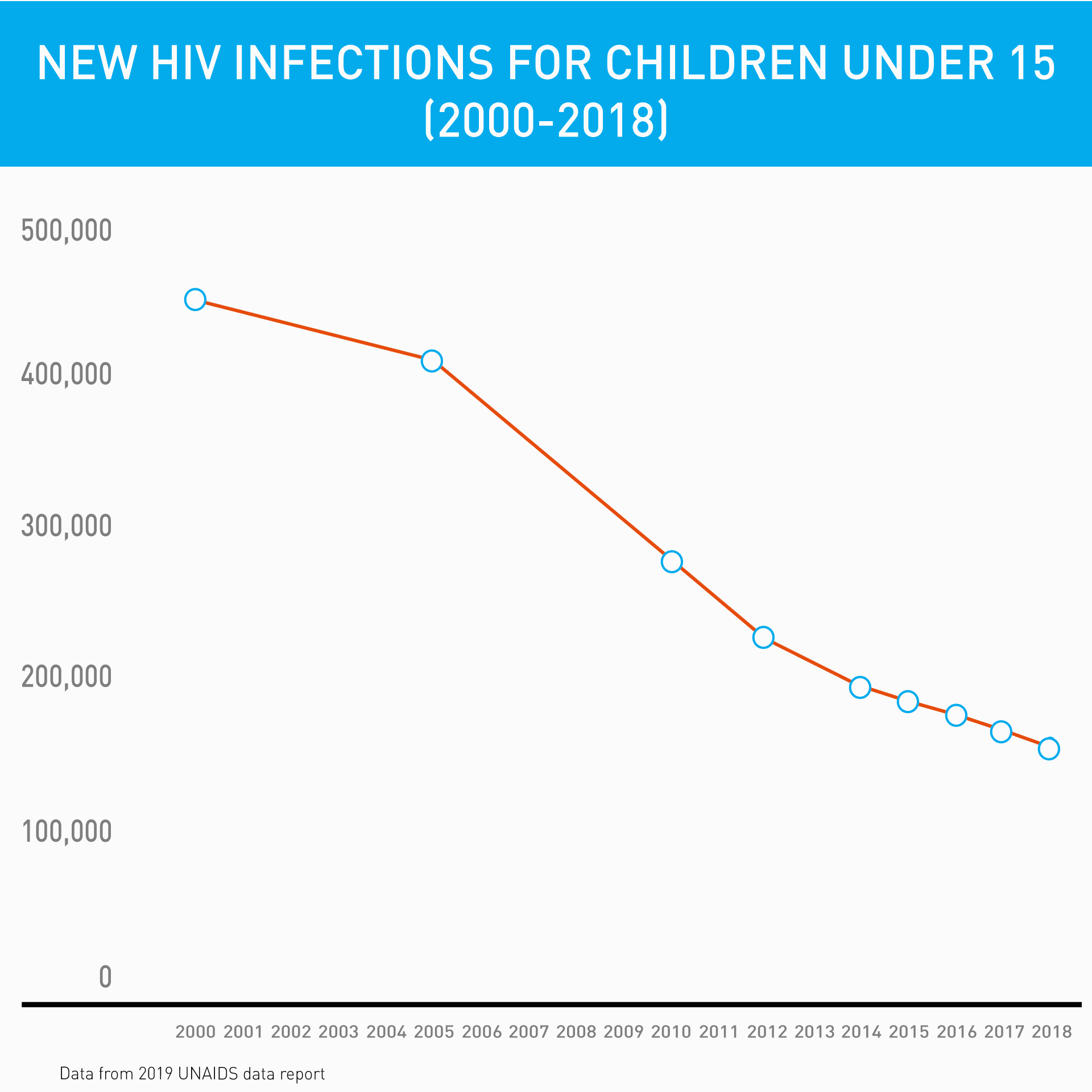
Despite the success of efforts to prevent mother-to-child transmission of HIV, children are still being born with HIV. There are some two million children (<15 years) living with the disease, the majority of them in sub-Saharan Africa.
Children with HIV are vulnerable. Without treatment, 50% of kids with HIV will die before they turn two, and 80% will die before they are five years old. In 2018, about 100,000 children died due to AIDS-related illnesses and 160,000 kids were infected, the vast majority through mother-to-child transmission. Having children tested and put on treatment early saves lives.

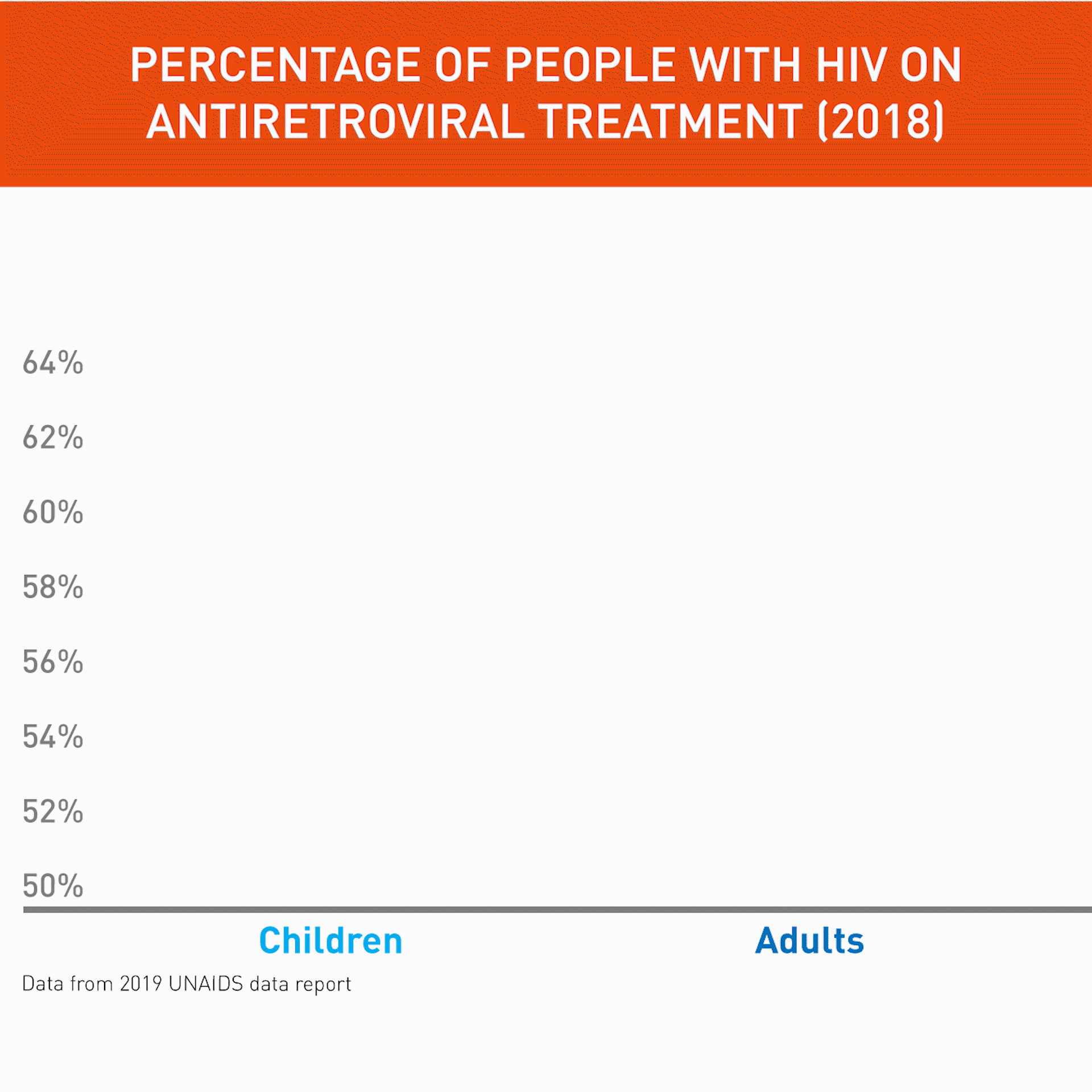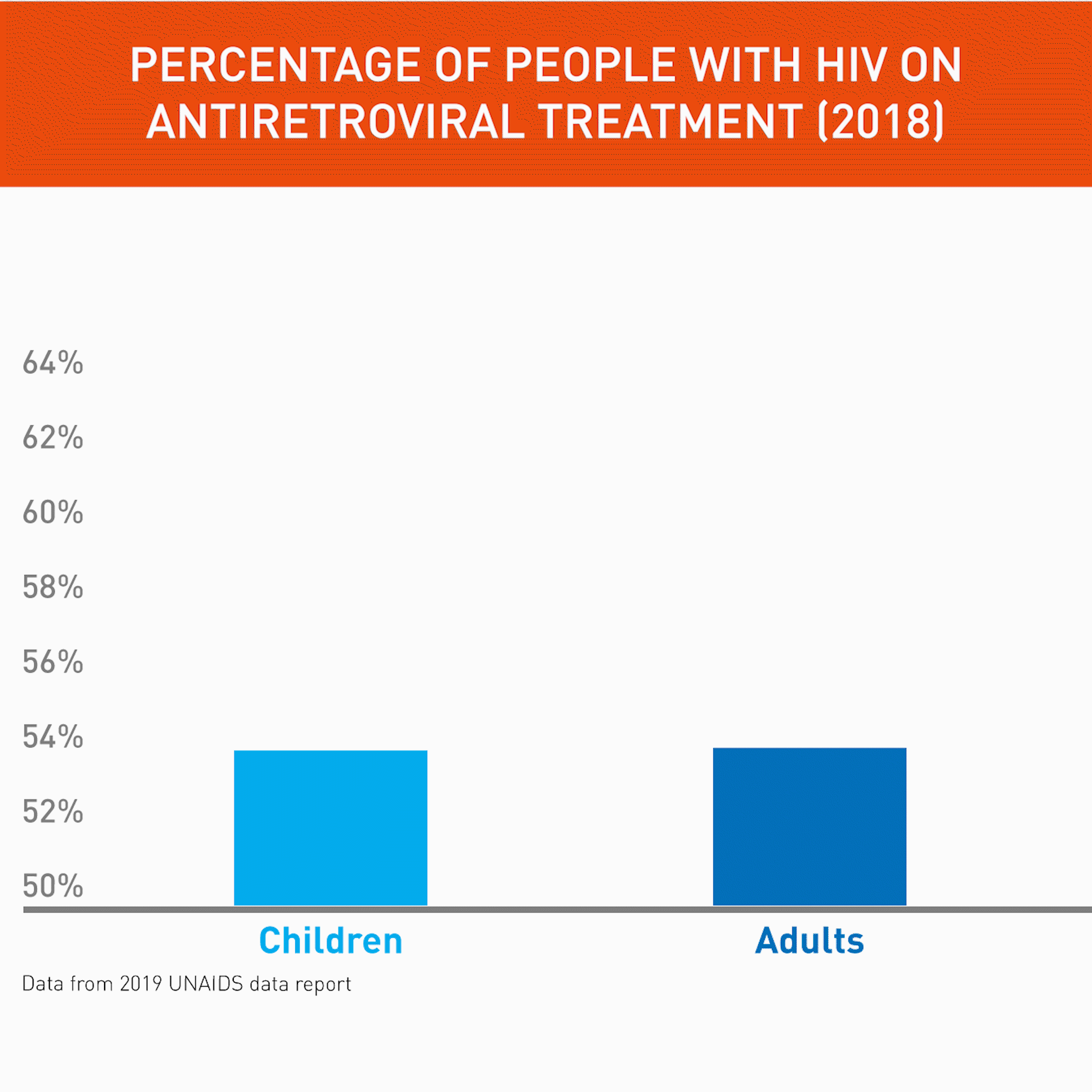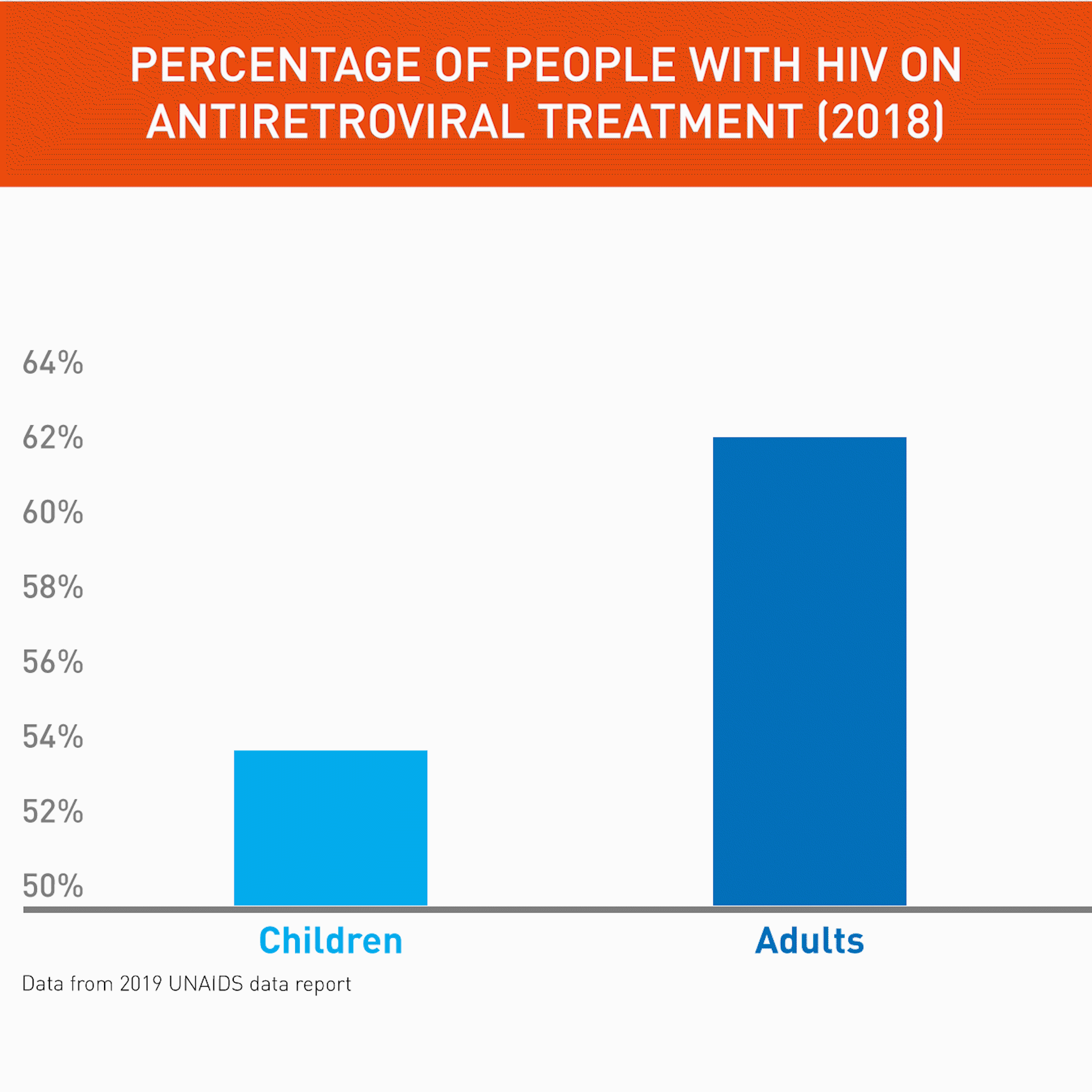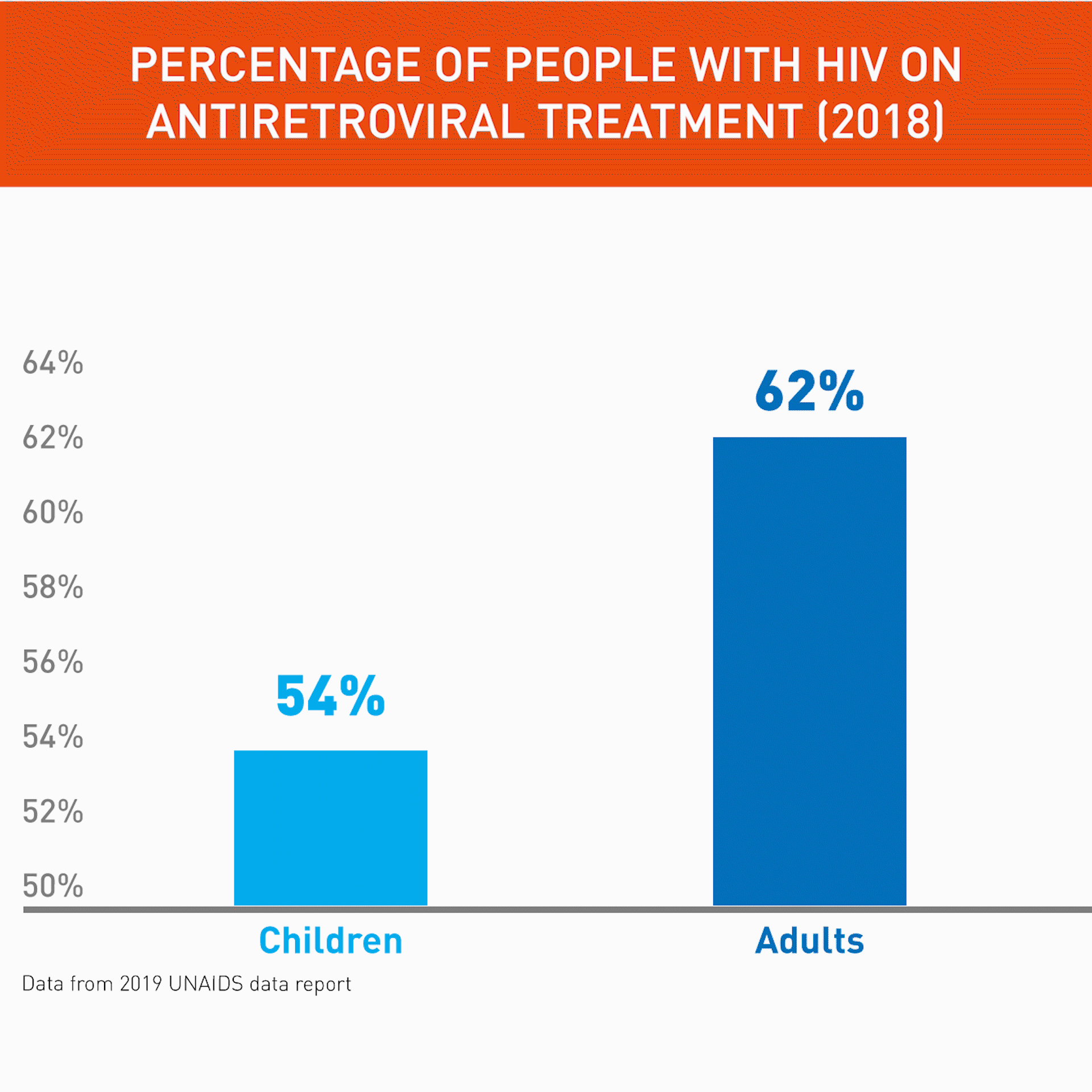
The world has seen tremendous advances in care for adults living with HIV. In 2018, some 62% of all people with HIV had access to treatment. But the figure is lower for kids. In 2018, only 54% of children living with HIV received antiretroviral treatment (ART) – in West and Central Africa it is even less, with 28% of children on ART.
Why are there fewer children on treatment?
To begin with, low coverage of early infant diagnosis (EID) has been one enduring obstacle. The WHO recommends that all HIV-exposed infants (children of those living with HIV) receive a virologic test for HIV within two months of birth, but less than 60% had access to these tests in 2018. Rapid saliva or blood tests won’t work on babies, as their blood contains their mother’s antibodies for up to 18 months after birth. Infants born to mothers with HIV need specialized virological testing using nucleic acid testing technologies. Until recently, these technologies have been available only in central laboratories.

Large EID machine in Uganda's capital, Kampala
Large EID machine in Uganda's capital, Kampala
Ruth, Mbarara's mother, lives relatively close to her hospital. But many mothers live tremendous distances from their hospitals, and transport can be a challenge. Caregivers are often single mothers or grandmothers who are sole breadwinners for their families. For mothers like Ruth, taking 1-2 days off from work to take their children for diagnosis or treatment can create significant financial hardship.
Meanwhile, mothers often board boda bodas with their children – a dangerous mode of transportation in a country where road accidents are a major killer.

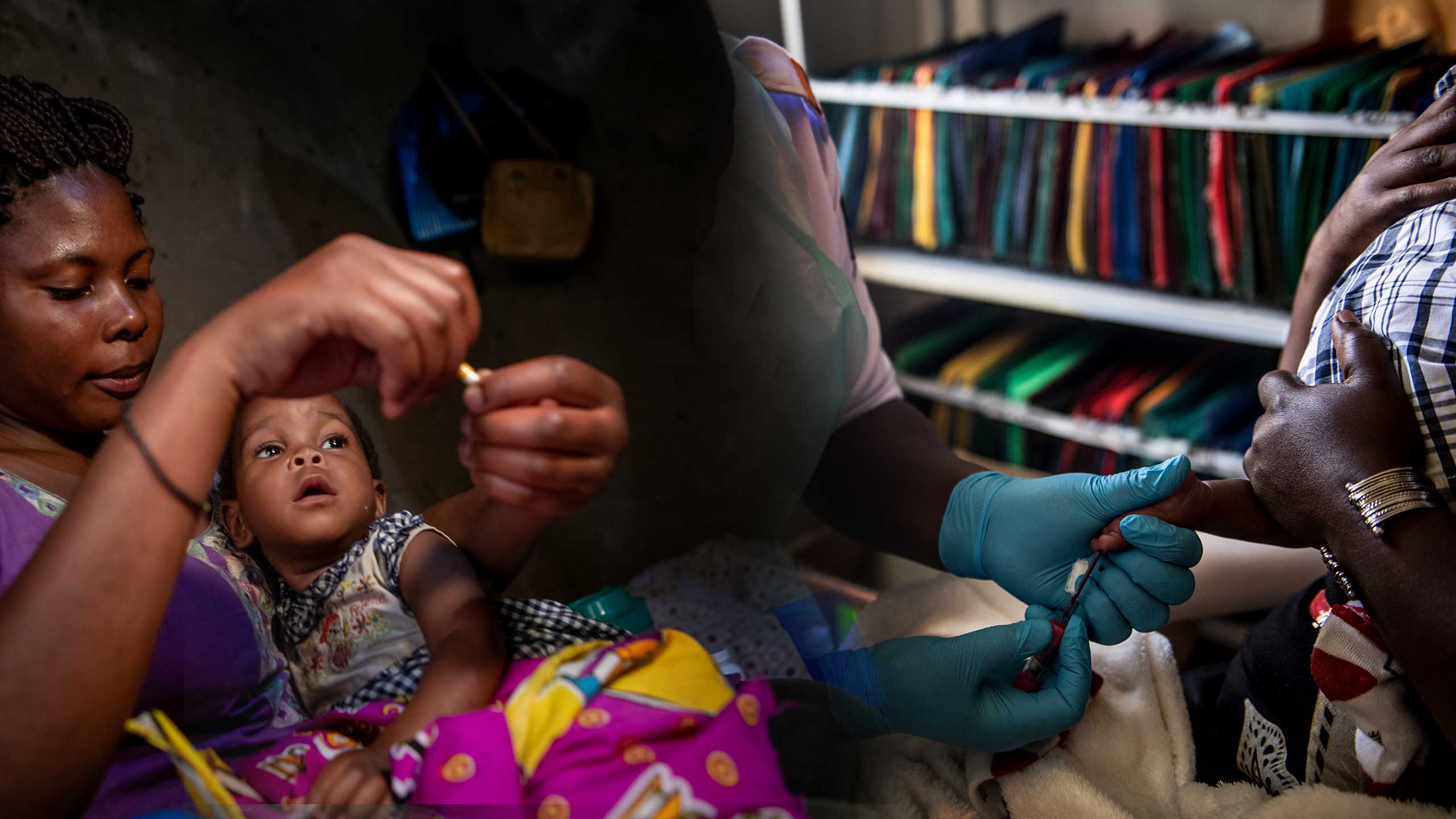
Before the introduction of innovative point-of-care diagnostic machines, test results often had to be sent to centralized laboratories, taking up to 90 days for caregivers to receive their child's test result, if they received it at all. Families were already travelling long distances from their communities to access care – imagine the burden on them to return to the hospital for a test result. Many patients were simply “lost to follow-up.”
To make matters worse, for too long the only WHO-recommended HIV treatments for kids were difficult to take, or foul-tasting. The most recent formulation available for infants and young children comes in the form of a syrup that tastes so awful, children often throw it back up. It contains more than 40% alcohol and requires refrigeration, making it difficult to store – and families without electricity must bury it in soil or sand to keep it cool.

A Kenyan mother with syrups that she keeps in a bucket with sand to keep cool
A Kenyan mother with syrups that she keeps in a bucket with sand to keep cool
Solutions
Two stories in Mbarara show how new technologies are responding to these medical needs, and leading to healthier children.
Testing innovations – Hajara's story

A few hours' car-ride away from Mbarara hospital past hills and dusty roads, Hajara starts her day with her two children, Nuriati and Musa. Living with her two children and her mother, Hajara provides for the family by baking bricks and harvesting matooke (East African bananas). Brick-making is an arduous task, requiring the maker to carry a large amount of water, dig soil from the ground, and then mould and bake the bricks in high heat. Although this task is physically demanding, Hajara is happy to do the work.

On a typical day, Musa and Nuriati can be found playing football with children from nearby houses. Nuriati was diagnosed with HIV and has been on treatment since her diagnosis. She is now growing up healthy.
Hajara visited Mbarara hospital when Nuriati fell sick and went to the hospital’s malnutrition unit. While Nuriati was being treated for malnutrition, doctors tested both Hajara and Nuriati for HIV. They were both positive. Testing children in inpatient paediatric wards, nutrition wards, and other sites that are not included in traditional HIV testing sites – also known as "alternative entry point testing" – allows for the diagnosis of children with HIV who may not have been tested otherwise. It allows for more children to be identified and put on life-saving treatment.

But the innovation goes further. Nuriati was tested at the malnutrition unit with a “point-of-care” early infant diagnosis machine, which uses advanced technology to analyze DNA in a blood sample.
Nuriati's blood was taken, then transported to a lab within Mbarara hospital, less than 10-minutes' walking distance from the malnutrition unit. The sample was then loaded on a cartridge-based point-of-care machine called GeneXpert, which returned results shortly. Hajara was informed Nuriati was positive on the same day, and her daughter was put on treatment immediately.
'Getting the results in such a short time made me very happy. At our local clinic here, there is no machine that gives immediate results. We received our medication very quickly.'
A new wave of similar point-of-care machines is now bringing this technology closer to patients, in Uganda and beyond. They are being assessed by regulatory authorities and introduced into countries like Uganda with great effect, complementing national laboratory networks.

Thanks to the faster turnaround times for test results enabled by point-of-care early infant diagnosis, children can be put onto treatment quickly, even on the same day they are diagnosed. This is a key step towards closing the gap between testing and treatment of children with HIV.
'My dream for my children is that I want to look after them, to educate them so that one day they might work in a hospital.'


Better treatment for children - Ruth's Story

Back in Ruth’s house outside of Mbarara, her children are benefitting from another innovation that Mbarara hospital has played a role in advancing. They are on a ‘2-in-1’ formulation containing two WHO-recommended treatments, lopinavir and ritonavir. Coming in the form of small pellets, this treatment is easily sprinkled on milk or soft food and must be taken with two other drugs that children with HIV need.
Joanita doesn’t have any issues swallowing pills, so she swallows the large pills that are available for kids her age. But Lighton is too young to swallow pills, so she is on the 2-in-1s. Ruth gives Lighton the pellets, sprinkling them on food.
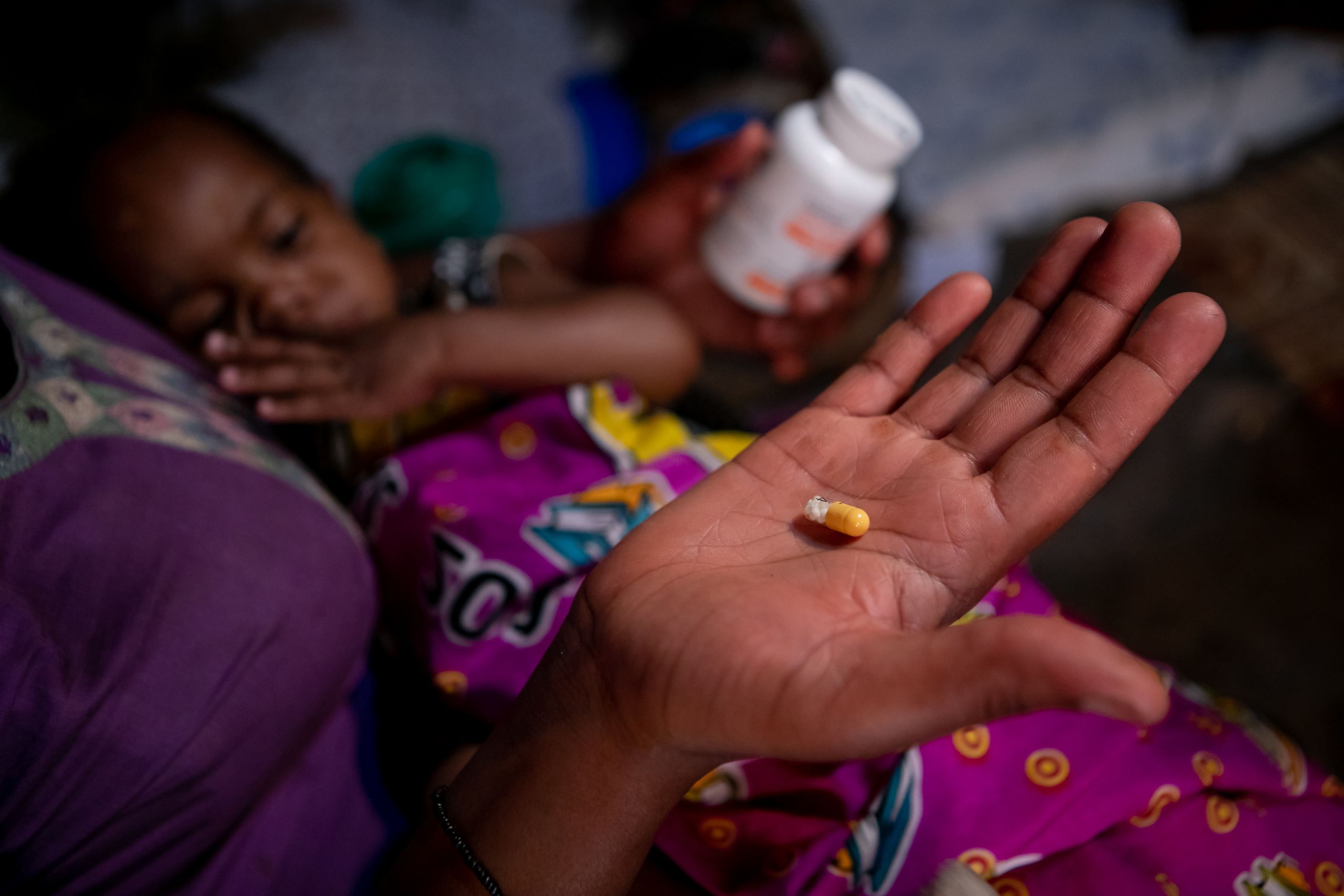
The 2-in-1 was approved by the U.S Food and Drug Administration in 2015 and DNDi then ran a large study in Uganda, Kenya, and Tanzania, including over 1,000 children who received the 2-in-1 formulation. Mbarara hospital was one of the sites for this large study. After interim results were released, the Ugandan government scaled up access to the treatment, reaching hospitals and clinics across the country.
Lighton is able to swallow the pellets, but they still have a bitter taste.
What children need is a “taste-masked” treatment that contains all four medicines recommended by the WHO in one capsule. DNDi and Cipla are now developing a 4-in-1 fixed-dose combination that tastes sweet and doesn’t require refrigeration, and comes in the form of granules that are even smaller than the 2-in-1 pellets.
If approved by the FDA next year, DNDi hopes that the 4-in-1 can be scaled up quickly to replace sub-optimal and difficult-to-administer treatments and give a needed boost to efforts to accelerate early diagnosis and treatment of children with HIV.


For more resources visit:
Children, HIV and AIDS: Global Snapshot 2019

Photos & videos by
Karin Schermbrucker - Slingshot
Rob Schermbrucker - Slingshot
Neil Brandvold
Mariella Furrer
DK Lee - DNDi

These projects are made possible thanks to Unitaid's support.

Unitaid accelerates access to innovation so that critical health products can reach the people who need them.




